why is an mrna copy made of the dna
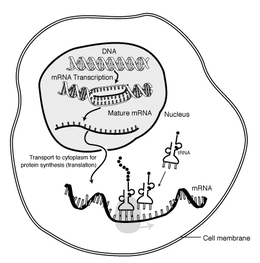
In molecular biology, messenger ribonucleic acid (template RNA) is a single-stranded molecule of RNA that corresponds to the genetical sequence of a gene, and is register away a ribosome in the process of synthesizing a protein.
mRNA is created during the process of transcription, where an enzyme (RNA polymerase) converts the cistron into primary copy mRNA (also titled pre-mRNA). This pre-informational RNA usually still contains introns, regions that will non go on to code for the final exam amino acidulent sequence. These are abstracted in the process of RNA splicing, going away only when exons, regions that will encode the protein. This exon sequence constitutes mature mRNA. Mature mRNA is then read by the ribosome, and, utilising amino acids carried by transfer RNA (tRNA), the ribosome creates the protein. This process is proverbial as translation. All of these processes form part of the central dogma of molecular biology, which describes the flowing of genetic information in a biologic system.
Every bit in DNA, genetic selective information in messenger RNA is contained in the chronological sequence of nucleotides, which are arranged into codons consisting of trine ribonucleotides each. Each codon codes for a specific amino acid, except the stop codons, which give the sac protein synthesis. The version of codons into amino acids requires two strange types of RNA: transfer RNA, which recognizes the codon and provides the like amino acid, and ribosomal RNA (rRNA), the inner component of the ribosome's protein-manufacturing machinery.
The idea of mRNA was first conceived away Sydney Brenner and Francis Henry Compton Crick on 15 April 1960 at King's College, Cambridge, while François Jacob was telling them about a recent try out conducted aside Chester Alan Arthur Pardee, himself, and Jacques Monod.[1] With Crick's encouragement, Brenner and Jacob immediately set out to test this new surmisal, and they reached bent Matthew Meselson at the California Institute of Applied science.[1] During the summer of 1960, Brenner, Jacob, and Meselson conducted an experiment in Meselson's laboratory at Caltech which established the existence of mRNA.[1] That fall, Jacob and Jacques Monod coined the name "messenger RNA" and developed the maiden theoretical framework to excuse its function.[1] In February 1961, Saint James the Apostle Watson revealed that his enquiry group was right behind them with a similar experiment in roughly the same direction; Brenner and the others in agreement to Watson's call for to postponement publishing of their explore findings.[1] As a result, the Brenner and Watson articles were published simultaneously in the aforementioned issue of Nature in Crataegus oxycantha 1961, patc that same month, Francois Jacob and Monod published their theoretical framework for messenger RNA in the Journal of Unit Biology.[1]
Synthesis, processing and part
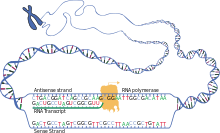
Ribonucleic acid polymerase transcribes a DNA strand to form mRNA
The brief existence of an mRNA molecule begins with transcription, and ultimately ends in degradation. During its life, an mRNA molecule may also be processed, edited, and transported prior to interlingual rendition. Eukaryotic mRNA molecules often need across-the-board processing and transport, while prokaryotic mRNA molecules do not. A molecule of eukaryotic mRNA and the proteins surrounding it are together called a messenger RNP.
Arranging
Transcription is when RNA is copied from DNA. During transcription, RNA polymerase makes a copy of a gene from the DNA to informational RNA as needed. This process differs slimly in eukaryotes and prokaryotes. One notable difference is that prokaryotic RNA polymerase associates with DNA-processing enzymes during written text soh that processing can go along during transcription. Thence, this causes the new mRNA strand to become double stranded by producing a complementary strand titled the tRNA string, which when combined are unable to grade structures from fundament-coupling. Moreover, the template for mRNA is the complementary color fibril of tRNA, which is identical in sequence to the anticodon sequence that the DNA binds to. The short-lived, unprocessed or partially processed product is termed precursor mRNA, OR pre-messenger RNA; once completely processed, it is termed mature mRNA.
Eucaryotic pre-informational RNA processing

DNA gene is transcribed to pre-mRNA, which is then computerized to contour a mature mRNA, and and then lastly translated by a ribosome to a protein
Processing of mRNA differs greatly among eukaryotes, bacteria, and archaea. Non-eukaryotic template RNA is, in essence, mature upon transcription and requires atomic number 102 processing, except in rare cases.[2] Eukaryotic pre-mRNA, withal, requires several processing steps before its transport to the cytol and its displacement away the ribosome.
Splicing
The extensive processing of eukaryotic pre-mRNA that leads to the mature mRNA is the RNA splicing, a mechanism away which introns operating room outrons (non-coding regions) are removed and exons (steganography regions) are joined together.
5' cap addition
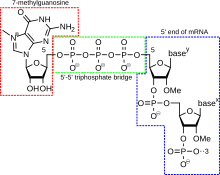
A 5' cap (also termed an RNA ceiling, an RNA 7-methylguanosine cap, or an RNA m7G cap) is a modified G nucleotide that has been added to the "front" or 5' closing of a eucaryotic messenger RNA shortly after the take up of transcription. The 5' capital consists of a terminal 7-methylguanosine residue that is linked through and through a 5'-5'-triphosphate bond to the first transcribed nucleotide. Its presence is critical for recognition away the ribosome and protection from RNases.
Cap addition is coupled to transcription, and occurs CO-transcriptionally, such that each influences the new. Presently afterward the start of recording, the 5' end of the mRNA being synthesized is paperbacked by a pileus-synthesizing complex joint with RNA polymerase. This enzymatic complex catalyzes the chemical reactions that are needful for template RNA capping. Synthesis return as a multi-step biochemical reaction.
Editing
In some instances, an mRNA leave be altered, changing the nucleotide composition of that mRNA. An example in humans is the apolipoprotein B informational RNA, which is edited in some tissues, but not others. The editing creates an early period codon, which, upon translation, produces a shorter protein.
Polyadenylation

Polyadenylation is the covalent linkage of a polyadenylyl moiety to a template RNA particle. In eukaryotic organisms near informational RNA (mRNA) molecules are polyadenylated at the 3' cease, but recent studies have shown that short stretches of uridine (oligouridylation) are also common.[3] The poly(A) tail and the protein bound to it help in protecting mRNA from degradation by exonucleases. Polyadenylation is also important for written text termination, export of the mRNA from the nucleus, and translation. messenger RNA can also be polyadenylated in being organisms, where poly(A) tails act to facilitate, rather than impede, exonucleolytic degradation.
Polyadenylation occurs during and/operating theatre immediately after written text of DNA into Ribonucleic acid. After transcription has been over, the mRNA chain is cleaved through the action of an endonuclease complex associated with RNA polymerase. After the mRNA has been cleaved, around 250 adenosine residues are added to the free 3' end at the cleavage site. This chemical reaction is catalyzed by polyadenylate polymerase. Even as in alternative splicing, in that location give notice exist more than one polyadenylation variate of an mRNA.
Polyadenylation website mutations also occur. The primary Ribonucleic acid transcript of a cistron is cleaved at the poly-A addition site, and 100–200 A's are added to the 3' end of the RNA. If this situation is altered, an abnormally long and unstable mRNA construct will be perfected.
Transport
Another difference between eukaryotes and prokaryotes is mRNA transport. Because eukaryotic arrangement and translation is compartmentally separated, eucaryotic mRNAs must be exported from the nucleus to the cytoplasm—a process that may be regulated past different signaling pathways.[4] Mature mRNAs are recognized by their processed modifications and then exported through and through the nuclear rive by binding to the cap-binding proteins CBP20 and CBP80,[5] as well American Samoa the transcription/export hard (TREX).[6] [7] Multiple mRNA exportation pathways have been known in eukaryotes.[8]
In spatially complex cells, some mRNAs are transported to particular subcellular destinations. In mature neurons, predestined mRNA are transported from the soma to dendrites. One internet site of mRNA translation is at polyribosomes selectively decentralized beneath synapses.[9] The mRNA for Arc/Arg3.1 is induced by synaptic activity and localizes selectively nearly brisk synapses supported on signals generated by NMDA receptors.[10] Another mRNAs also get into dendrites in response to external stimuli, such as β-actin mRNA.[11] Upon export from the nucleus, actin informational RNA associates with ZBP1 and the 40S subunit. The complex is bound by a efferent protein and is transported to the butt location (neurite telephone extension) along the cytoskeleton. Eventually ZBP1 is phosphorylated by Src in order for translation to live initiated.[12] In developing neurons, mRNAs are also transported into growing axons and especially growth cones. Many mRNAs are marked with sol-called "zip codes," which target their transport to a specific location.[13]
Displacement
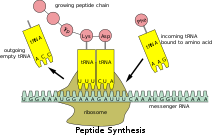
Translation of mRNA to protein
Because prokaryotic mRNA does not need to comprise processed or transported, translation by the ribosome can begin immediately after the end of transcription. Therefore, it can be said that procaryotic translation is conjugate to transcription and occurs atomic number 27-transcriptionally.
Eukaryotic mRNA that has been treated and transported to the cytoplasm (i.e., developed mRNA) can then follow translated by the ribosome. Translation may occur at ribosomes free-floating in the cytol, or directed to the endoplasmic reticulum by the signal recognition particle. Therefore, unequal in prokaryotes, eukaryotic translation is not directly conjugate to recording. It is even possible in some contexts that reduced mRNA levels are attended by increased protein levels, as has been observed for template RNA/protein levels of EEF1A1 in breast Cancer the Crab.[14] [ non-primary beginning needed ]
Social organisation

Coding regions
Coding regions are composed of codons, which are decoded and translated into proteins by the ribosome; in eukaryotes usually into one and in prokaryotes usually into several. Coding regions begin with the start codon and end with a ba codon. In national, the start codon is an AUG threesome and the stop codon is UAG ("yellow-brown"), UAA ("ochre"), or UGA ("opal"). The coding regions tend to be stabilised past internal root word pairs, this impedes degradation.[15] [16] In addition to being protein-coding, portions of steganography regions English hawthorn serve as regulatory sequences in the pre-mRNA Eastern Samoa exonic splicing enhancers or exonic splicing silencers.
Untranslated regions

Universal construction of eukaryotic mRNA, showing the structure of the 5' and 3' UTRs.
Untranslated regions (UTRs) are sections of the mRNA in front the showtime codon and after the layover codon that are non translated, termed the basketball team prime untranslated region (5' UTR) and three flus untranslated region (3' UTR), respectively. These regions are transcribed with the coding region and thence are exonic as they are present in the mature informational RNA. Several roles in gene expression have been attributed to the untranslated regions, including mRNA stability, mRNA localization, and change of location efficiency. The ability of a UTR to perform these functions depends on the sequence of the UTR and fire differ between mRNAs. Genetic variants in 3' UTR have also been implicated in disease susceptibleness because of the change in Ribonucleic acid structure and protein translation.[17]
The stability of mRNAs may be controlled by the 5' UTR and/or 3' UTR referable variable kinship for RNA harmful enzymes titled ribonucleases and for ancillary proteins that nates promote or inhibit RNA debasement. (See also, C-rich constancy ingredient.)
Translational efficiency, including sometimes the complete inhibition of translation, can be controlled by UTRs. Proteins that bind to either the 3' or 5' UTR may touch translation by influencing the ribosome's ability to bind to the mRNA. MicroRNAs bound to the 3' UTR also May affect translational efficiency operating theater mRNA stableness.
Cytoplasmic localization of messenger RNA is thought to represent a function of the 3' UTR. Proteins that are necessary in a particular region of the cell can also exist translated there; in such a case, the 3' UTR May contain sequences that allow the transcript to be localized to this region for interlingual rendition.
Some of the elements contained in untranslated regions form a device characteristic secondary structure when transcribed into RNA. These structural messenger RNA elements are involved in regulation the mRNA. About, so much as the SECIS element, are targets for proteins to obligate. One class of mRNA component, the riboswitches, directly bind small molecules, changing their fold to alter levels of transcription or translation. In these cases, the mRNA regulates itself.
Poly(A) tail
The 3' poly(A) tail is a long sequence of A nucleotides (often several one hundred) added to the 3' end of the pre-mRNA. This tag along promotes exportation from the nucleus and translation, and protects the messenger RNA from abjection.
Monocistronic versus polycistronic mRNA
An mRNA molecule is said to be monocistronic when it contains the genetic information to translate only when a sui generis protein chain (polypeptide). This is the case for most of the eukaryotic mRNAs.[18] [19] On the other hand, polycistronic mRNA carries several ingenuous reading frames (ORFs), each of which is translated into a polypeptide. These polypeptides usually rich person a related function (they often are the subunits composing a final complex protein) and their coding chronological succession is grouped and regulated together in a regulatory region, containing a booster and an operator. Nearly of the mRNA found in bacterium and archaea is polycistronic,[18] every bit is the weak mitochondrial genome.[20] Dicistronic or bicistronic mRNA encodes exclusively two proteins.
mRNA circularization

messenger RNA circularisation and regulation
In eukaryotes mRNA molecules form circular structures attributable an interaction between the eIF4E and poly(A)-cover protein, which both bind to eIF4G, forming an mRNA-protein-messenger RNA bridge.[21] Circularisation is sentiment to promote cycling of ribosomes on the mRNA directive to time-efficient translation, and may also work to ensure single intact mRNA are translated (partially degraded template RNA characteristically have nobelium m7G cap, Beaver State no poly-A tail).[22]
Strange mechanisms for circularization live, particularly in virus mRNA. Poliovirus template RNA uses a cloverleaf part towards its 5' end to bind PCBP2, which binds poly(A)-binding protein, forming the everyday template RNA-protein-mRNA circle. Barleycorn yellow dwarf computer virus has binding between mRNA segments on its 5' end and 3' end (called kissing prow loops), circularizing the mRNA without any proteins involved.
RNA virus genomes (the + strands of which are translated as mRNA) are also ordinarily circularized.[ citation needed ] During genome replication the circularization acts to enhance genome replication speeds, cycling viral RNA-dependent RNA polymerase much the same as the ribosome is hypothesized to cycle.
Degradation
Different mRNAs within the same cell have different lifetimes (stabilities). In bacterial cells, individual mRNAs tooshie survive from seconds to more than an hr. However, the lifetime averages between 1 and 3 minutes, qualification microorganism mRNA much less stable than eukaryotic mRNA.[23] In mammalian cells, messenger RNA lifetimes cooking stove from individual minutes to years.[24] The greater the stableness of an mRNA the more protein may be produced from that messenger RNA. The constricted lifetime of mRNA enables a jail cell to alter protein synthesis apace in response to its changing inevitably. There are many mechanisms that lead to the destruction of an mRNA, some of which are delineated below.
Prokaryotic template RNA degradation
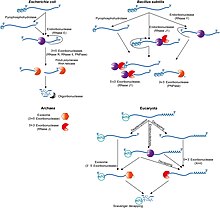
Overview of mRNA decay pathways in the different life domains.
Generally, in prokaryotes the lifetime of mRNA is much shorter than in eukaryotes. Prokaryotes degrade messages by using a combination of ribonucleases, including endonucleases, 3' exonucleases, and 5' exonucleases. In about instances, small RNA molecules (sRNA) tens to hundreds of nucleotides long can stimulate the debasement of specific mRNAs by base-pairing with complementary sequences and facilitating ribonuclease segmentation by RNase III. Information technology was recently shown that bacteria also have a sort of 5' cap consisting of a triphosphate along the 5' ending.[25] Remotion of two of the phosphates leaves a 5' monophosphate, causing the message to be sacked by the exonuclease RNase J, which degrades 5' to 3'.
Eukaryotic mRNA turnover
Inside eukaryotic cells, there is a balance 'tween the processes of displacement and mRNA crumble. Messages that are being actively translated are bound by ribosomes, the eukaryotic initiation factors eIF-4E and eIF-4G, and poly(A)-binding protein. eIF-4E and eIF-4G stop the decapping enzyme (DCP2), and poly(A)-binding protein blocks the exosome complex, protecting the ends of the message. The balance 'tween translation and decay is reflected in the size up and abundance of living substance structures titled P-bodies[26] The poly(A) tail of the mRNA is shortened by technical exonucleases that are targeted to specific messenger RNAs aside a combination of Commonwealth of Independent States-regulatory sequences on the RNA and trans-acting Ribonucleic acid-binding proteins. Poly(A) tail remotion is thought to disrupt the circular structure of the message and destabilize the cap binding complex. The message is then subject to degradation away either the exosome complex surgery the decapping complex. In this way, translationally inactive messages can be war-torn chop-chop, while active messages remain intact. The mechanics past which translation stops and the message is handed-off to decay complexes is not understood in particular.
AU-full element decomposition
The presence of AU-rich elements in some mammalian mRNAs tends to destabilize those transcripts through the action of cellular proteins that tie these sequences and stimulate poly(A) tail removal. Loss of the poly(A) tail is thought to promote mRNA degradation by facilitating attack by both the exosome complex[27] and the decapping complex.[28] Rapid mRNA degradation via AU-rich elements is a critical mechanism for preventing the overrun of influential cytokines much as tumour sphacelus factor (TNF) and granulocyte-macrophage settlement stimulative factor (GM-CSF).[29] AU-plentiful elements as wel regulate the biogenesis of early-oncogenic transcription factors like c-Jun and c-Fos.[30]
Nonsense-mediated decay
Eukaryotic messages are field of study to surveillance past nonsense-mediated decay (NMD), which checks for the presence of premature stop codons (nonsense codons) in the message. These can arise via incomplete splice, V(D)J recombination in the adaptive condition arrangement, mutations in DNA, transcription errors, leaky scanning by the ribosome causation a frame shift, and different causes. Detecting of a premature stop codon triggers mRNA degradation by 5' decapping, 3' poly(A) chase removal, or endonucleolytic segmentation.[31]
Small interfering Ribonucleic acid (siRNA)
In metazoans, small interfering RNAs (siRNAs) processed away Dicer are incorporated into a complex known as the RNA-induced silencing complex or RISC. This complex contains an endonuclease that cleaves perfectly additive messages to which the siRNA binds. The resultant mRNA fragments are and so destroyed by exonucleases. siRNA is commonly used in laboratories to impede the subprogram of genes in cell culture. It is mentation to be part of the innate immune system as a defence reaction against double-stranded RNA viruses.[32]
MicroRNA (miRNA)
MicroRNAs (miRNAs) are small RNAs that typically are partially complementary to sequences in metazoan courier RNAs.[33] [34] Bandaging of a miRNA to a message can repress version of that message and quicken poly(A) tail removal, thereby hastening mRNA debasement. The mechanics of action of miRNAs is the field of active research.[35] [36]
Other decay mechanisms
There are other slipway by which messages force out be degraded, including non-stop decay and silencing past Piwi-interacting Ribonucleic acid (piRNA), among others.
Applications
The administration of a nucleoside-modified messenger RNA sequence can cause a cell to make a protein, which in plow could now treat a disease or could function as a vaccine; more indirectly the protein could drive an endogenous stem cellphone to differentiate in a desired way.[37] [38]
The primary challenges of RNA therapy center on delivering the RNA to the appropriate cells.[39] Challenges let in the fact that naked RNA sequences naturally degrade after cooking; they may trigger the torso's immune system to attack them as an invader; and they are impermeable to the cell membrane.[38] Once inside the cell, they must then leave the cell's transport mechanism to rent action within the cytol, which houses the requisite ribosomes.[37]
Overcoming these challenges, informational RNA as a therapeutic was first put impudent in 1989 "subsequently the growing of a generally applicable in vitro transfection proficiency."[40] In the 1990s, mRNA vaccines for personalized cancer cause been developed, relying on non-nucleoside modified mRNA. mRNA based therapies continue to be investigated equally a method acting of treatment or therapy for both Cancer too as machine-resistant, metabolic, and respiratory inflammatory diseases. Gene editing therapies such American Samoa CRISPR may also welfare from using mRNA to induce cells to make the desired Cas protein.[41]
Since the 2010s, Ribonucleic acid vaccines and other RNA therapeutics have been considered to be "a new class of drugs."[42] The starting time mRNA-based vaccines received out-of-bounds authorization and were rolled unsuccessful across the world during the COVID-19 pandemic by Pfizer–BioNTech COVID-19 vaccine and Moderna, for object lesson.[43] [44]
See also
- GeneCalling, an mRNA profiling technology
- Missense messenger RNA
- mRNA expose
- mRNA surveillance
- Transcriptome, the sum of all Ribonucleic acid in a cadre
References
- ^ a b c d e f Cobb M (29 June 2015). "Who discovered messenger RNA?". Present-day Biology. 25 (13): R526–R532. Interior:10.1016/j.cub.2015.05.032. PMID 26126273.
- ^ Watson JD (February 22, 2013). Molecular Biological science of the Gene, 7th variant. Pearson Higher Ed USA. ISBN9780321851499.
- ^ Choi YS, Patena W, Leavitt AD, McManus MT (March 2012). "Widespread RNA 3'-end oligouridylation in mammals". RNA (Greater New York, N.Y.). 18 (3): 394–401. doi:10.1261/rna.029306.111. PMC3285928. PMID 22291204.
- ^ Quaresma AJ, Sievert R, Nickerson JA (April 2013). "Regulation of mRNA exportation by the PI3 kinase/AKT signal transduction pathway". Molecular Biology of the Cell. 24 (8): 1208–1221. Interior Department:10.1091/mbc.E12-06-0450. PMC3623641. PMID 23427269.
- ^ Kierzkowski D, Kmieciak M, Piontek P, Wojtaszek P, Szweykowska-Kulinska Z, Jarmolowski A (Sept 2009). "The Arabidopsis CBP20 targets the cap-binding multiplex to the nucleus, and is stabilized by CBP80". The Plant Journal. 59 (5): 814–825. doi:10.1111/j.1365-313X.2009.03915.x. PMID 19453442.
- ^ Strässer K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, Rondón AG, Aguilera A, Struhl K, Reed R, Hurt E (May 2002). "TREX is a conserved hard coupling transcription with courier RNA exportation". Nature. 417 (6886): 304–308. Bibcode:2002Natur.417..304S. doi:10.1038/nature746. PMID 11979277. S2CID 1112194.
- ^ Katahira J, Yoneda Y (27 October 2014). "Roles of the TREX complex in nuclear export of mRNA". RNA Biology. 6 (2): 149–152. Department of the Interior:10.4161/rna.6.2.8046. PMID 19229134.
- ^ Cenik C, Chua Hydrogen azide, Zhang H, Tarnawsky SP, Akef A, Derti A, Tasan M, Moore MJ, Palazzo AF, Roth FP (April 2011). "Genome analysis reveals interplay betwixt 5'UTR introns and cell organelle mRNA export for secretory and mitochondrial genes". PLOS Genetic science. 7 (4): e1001366. doi:10.1371/journal.pgen.1001366. PMC3077370. PMID 21533221.
- ^ Steward O, Levy en masse WB (March 1982). "Preferential localisatio of polyribosomes below the base of dendritic spines in granule cells of the dentate gyrus". The Journal of Neuroscience. 2 (3): 284–291. doi:10.1523/JNEUROSCI.02-03-00284.1982. PMC6564334. PMID 7062109.
- ^ Steward O, Worley PF (April 2001). "Selective targeting of newly synthesized Arc mRNA to nimble synapses requires NMDA sense organ activation". Nerve cell. 30 (1): 227–240. Department of the Interior:10.1016/s0896-6273(01)00275-6. PMID 11343657. S2CID 13395819.
- ^ Problem, Christy; James Eberwine (Dec 2001). "Localisation principle and translation of mRNA in dendrites and axons". Nature Reviews. Neuroscience. 2 (12): 889–898. doi:10.1038/35104069. PMID 11733796. S2CID 5275219.
- ^ Hüttelmaier S, Zenklusen D, Lederer M, Dictenberg J, Lorenz M, Meng X, et al. (November 2005). "Attribute regulation of beta-actin rendering by Src-dependent phosphorylation of ZBP1". Nature. 438 (7067): 512–5. Bibcode:2005Natur.438..512H. doi:10.1038/nature04115. PMID 16306994. S2CID 2453397.
- ^ Ainger K, Avossa D, Diana AS, Barry C, Barbarese E, Rachel Carson JH (September 1997). "Transport and localization principle elements in medull basic protein mRNA". The Journal of Cell Biology. 138 (5): 1077–1087. Department of the Interior:10.1083/jcb.138.5.1077. PMC2136761. PMID 9281585.
- ^ Lin CY, Beattie A, Baradaran B, Dray E, Duijf PH (September 2018). "Contradictory mRNA and protein misexpression of EEF1A1 in ductal tit carcinoma overdue to cell pedal ordinance and faveolate stress". Scientific Reports. 8 (1): 13904. Bibcode:2018NatSR...813904L. DoI:10.1038/s41598-018-32272-x. PMC6141510. PMID 30224719.
- ^ Shabalina SA, Ogurtsov AY, Spiridonov Na (2006). "A rhythmic pattern of informational RNA secondary structure created by the genetic code". Nucleic Acids Research. 34 (8): 2428–2437. doi:10.1093/nar/gkl287. PMC1458515. PMID 16682450.
- ^ Katz L, Burge CB (September 2003). "Widespread selection for local RNA secondary structure in coding regions of bacterial genes". Genome Research. 13 (9): 2042–2051. Interior:10.1101/gr.1257503. PMC403678. PMID 12952875.
- ^ Lu YF, Mauger DM, Goldstein DB, Urban TJ, Weeks KM, Bradrick SS (November 2015). "IFNL3 mRNA structure is remodeled by a functional non-coding polymorphism associated with hepatitis C virus headway". Scientific Reports. 5: 16037. Bibcode:2015NatSR...516037L. doi:10.1038/srep16037. PMC4631997. PMID 26531896.
- ^ a b Kozak M (March 1983). "Comparison of founding of protein synthesis in procaryotes, eucaryotes, and organelles". Microbiological Reviews. 47 (1): 1–45. doi:10.1128/MMBR.47.1.1-45.1983. PMC281560. PMID 6343825.
- ^ Niehrs C, Pollet N (December 1999). "Synexpression groups in eukaryotes". Nature. 402 (6761): 483–487. Bibcode:1999Natur.402..483N. Interior:10.1038/990025. PMID 10591207. S2CID 4349134.
- ^ Mercer TR, Neph S, Dinger ME, Joan Crawford J, Smith Momma, Shearwood AM, Haugen E, Bracken CP, Rackham O, Stamatoyannopoulos JA, Filipovska A, Mattick JS (August 2011). "The human mitochondrial transcriptome". Cell. 146 (4): 645–658. Department of the Interior:10.1016/j.jail cell.2011.06.051. PMC3160626. PMID 21854988.
- ^ H. G. Wells SE, Hillner PE, Vale RD, Sachs AB (July 1998). "Circularization of mRNA by eukaryotic translation initiation factors". Molecular Cell. 2 (1): 135–140. CiteSeerX10.1.1.320.5704. DoI:10.1016/S1097-2765(00)80122-7. PMID 9702200.
- ^ López-Lastra M, Rivas A, Barría MI (2005). "Protein synthesis in eukaryotes: the growing biological relevance of cap-independent translation initiation". Natural Explore. 38 (2–3): 121–146. doi:10.4067/S0716-97602005000200003. PMID 16238092.
- ^ Lewin B, Hans Adolf Krebs JE, Kilpatrick ST, Goldstein ES, eds. (2011). Lewin's genes X (10th ed.). Sudbury, Mass.: Mary Harris Jone and Bartlett. ISBN9780763766320. OCLC 456641931.
- ^ Yu J, Henry Kenneth Alfred Russell JE (September 2001). "Geomorphologic and functional depth psychology of an mRNP complex that mediates the upper stability of human explorative-globin mRNA". Molecular and Cellular Biology. 21 (17): 5879–5888. doi:10.1128/mcb.21.17.5879-5888.2001. PMC87307. PMID 11486027.
- ^ Deana A, Celesnik H, Belasco JG (January 2008). "The bacterial enzyme RppH triggers messenger Ribonucleic acid debasement by 5' pyrophosphate removal". Nature. 451 (7176): 355–358. Bibcode:2008Natur.451..355D. doi:10.1038/nature06475. PMID 18202662. S2CID 4321451.
- ^ Parker R, Sheth U (March 2007). "P bodies and the ascendency of mRNA translation and degradation". Molecular Cadre. 25 (5): 635–646. doi:10.1016/j.molcel.2007.02.011. PMID 17349952.
- ^ Chen CY, Gherzi R, Ong Atomic number 34, Chan EL, Raijmakers R, Pruijn GJ, Stoecklin G, Moroni C, Mann M, Karin M (November 2001). "AU binding proteins recruit the exosome to degrade ARE-containing mRNAs". Cell. 107 (4): 451–464. Interior:10.1016/S0092-8674(01)00578-5. PMID 11719186. S2CID 14817671.
- ^ Fenger-Grøn M, Fillman C, Norrild B, Lykke-Andersen J (December 2005). "Multiple processing body factors and the ARE bandaging protein TTP activate mRNA decapping". Molecular Cellular telephone. 20 (6): 905–915. Department of the Interior:10.1016/j.molcel.2005.10.031. PMID 16364915.
- ^ Shaw G, Kamen R (Honorable 1986). "A conserved AU sequence from the 3' untranslated part of GM-CSF mRNA mediates selective mRNA debasement". Jail cell. 46 (5): 659–667. doi:10.1016/0092-8674(86)90341-7. PMID 3488815. S2CID 40332253.
- ^ Chen CY, Shyu AB (November 1995). "AU-rich elements: delineation and importance in mRNA degradation". Trends in Biochemical Sciences. 20 (11): 465–470. doi:10.1016/S0968-0004(00)89102-1. PMID 8578590.
- ^ Isken O, Maquat LE (August 2007). "Caliber control of eucaryotic mRNA: safeguarding cells from supernormal mRNA function". Genes & Developing. 21 (15): 1833–1856. Interior:10.1101/gad.1566807. PMID 17671086.
- ^ Obbard Disc jockey, Gordon KH, Buck AH, Jiggins FM (January 2009). "The organic evolution of RNAi equally a defence against viruses and permutable elements". Philosophic Transactions of the Royal Society of London. Series B, Life Sciences. 364 (1513): 99–115. doi:10.1098/rstb.2008.0168. PMC2592633. PMID 18926973.
- ^ Robert E. Farrell, Jr. RNA Methodologies, 5th Edition. Academic Press, 2017
- ^ Brennecke J, Stark A, Russell RB, Cohen MS (March 2005). "Principles of microRNA-target recognition". PLOS Biology. 3 (3): e85. doi:10.1371/journal.pbio.0030085. PMC1043860. PMID 15723116.
- ^ Tasuku Honjo, Michael Reth, Andreas Radbruch, Frederick Altitude. Molecular Biology of B Cells, 2nd Edition. Academic Weightlift, 2014 (including "updated research on microRNAs")
- ^ Eulalio A, Huntzinger E, Nishihara T, Rehwinkel J, Fauser M, Izaurralde E (January 2009). "Deadenylation is a far-flung effect of miRNA regulation". Ribonucleic acid. 15 (1): 21–32. Interior Department:10.1261/rna.1399509. PMC2612776. PMID 19029310.
- ^ a b Hadj KA, Whitehead KA (12 September 2017). "Tools for interlingual rendition: not-viral materials for cure mRNA delivery". Nature Reviews Materials. 2 (10): 17056. Bibcode:2017NatRM...217056H. doi:10.1038/natrevmats.2017.56.
- ^ a b Gousseinov E, Kozlov M, Scanlan C (September 15, 2015). "RNA-Based Therapeutics and Vaccines". Genetic Engineering News.
- ^ Kaczmarek JC, Kowalski PS, Anderson DG (June 2017). "Advances in the livery of Ribonucleic acid therapeutics: from conception to clinical reality". Genome Medicament. 9 (1): 60. doi:10.1186/s13073-017-0450-0. PMC5485616. PMID 28655327.
- ^ Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ (November 2012). "Developing informational RNA-vaccine technologies". RNA Biology. 9 (11): 1319–30. doi:10.4161/rna.22269. PMC3597572. PMID 23064118.
- ^ Haridi R (2021-04-23). "The mRNA gyration: How COVID-19 hit fast-onwards on an experimental technology". New Atlas . Retrieved 2021-04-26 .
- ^ Kowalska J, Wypijewska del Nogal A, Darzynkiewicz ZM, Buck J, Nicola C, Kuhn AN, Lukaszewicz M, Zuberek J, Strenkowska M, Ziemniak M, Maciejczyk M, Bojarska E, Rhoads RE, Darzynkiewicz E, Sahin U, Jemielity J (2014), "mRNA-settled therapeutics–nonindustrial a new-sprung class of drugs.", Nature Reviews Drug Breakthrough, 13 (10), pp. 759–780, doi:10.1093/nar/gku757, PMC4176373, PMID 25150148
- ^ P. Polack, Fernando; Thomas, Stephen J.; Kitchin, St. Nicholas; Absalon, Judith; Gurtman, Alejandra; Lockhart, Sir Leslie Stephen; Perez, Bathroom L.; Pérez Marc, Gonzalo; Moreira, Edson D.; Zerbini, Cristiano; Bailey, George Herman Ruth; Swanson, Kena A.; Roychoudhury, Satrajit; Koury, Kenneth; Li, Ping; Kalina, Warren V.; Cooper, David; Frenck, Robert W.; Hammitt, Laura L.; Türeci, Özlem; Nell, Haylene; Schaefer, Axel; Ünal, Serhat; Tresnan, Dina B.; Mather, Susan; Dormitzer, Philip R.; Şahin, Uğur; Jansen, Kathrin U.; Gruber, William C. (2020), "Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine.", New England Journal of Medicine, 383 (27): 2603–15, Department of the Interior:10.1056/NEJMoa2034577, PMC7745181, PMID 33301246
- ^ Walsh, Edward E.; Frenck, Robert W.; Falsey, Ann R.; Kitchin, Saint Nicholas; Absalon, Judith; Gurtman, Alejandra; Lockhart, Stephen; Neuzil, Kathleen; Mulligan, Mark J.; Bailey, Ruth; Gloria May Josephine Svensson, Kena A.; Li, Ping; Koury, Kenneth; Kalina, Warren; Cooper, David; Fontes‑Garfias, Camila; Shi, Pei‑Yong; Türeci, Özlem; Tompkins, Kristin R.; Lyke, Kirsten E.; Raabe, Vanessa; Dormitzer, Duke of Edinburgh R.; Jansen, Kathrin U.; Şahin, Uğur; Gruber, William C. (2020), "Safety and Immunogenicity of Two Ribonucleic acid-Based Covid-19 Vaccinum Candidates.", New England Daybook of Medicine, 383 (25): 2439–50, doi:10.1056/NEJMoa2027906, PMC7583697, PMID 33053279
Foster reading
- Alsaweed M, Lai CT, Hartmann PE, Geddes DT, Kakulas F (February 2016). "Frail milk miRNAs primarily initiate from the exocrine secreter resulting in unique miRNA profiles of fractionated milk". Scientific Reports. 6 (1): 20680. Bibcode:2016NatSR...620680A. Interior:10.1038/srep20680. PMC4745068. PMID 26854194.
- Lillycrop Ka, Burdge GC (October 2012). "Epigenetic mechanisms linking early nutrition to elongate term health". Best Practice & Enquiry. Clinical Endocrinology & Metabolism. 26 (5): 667–676. doi:10.1016/j.beem.2012.03.009. PMID 22980048.
- Melnik BC, Kakulas F, Geddes DT, Hartmann PE, John SM, Carrera-Bastos P, Cordain L, Schmitz G (21 June 2016). "Milk miRNAs: simple nutrients or systemic operative regulators?". Sustenance & Metabolic process. 13 (1): 42. Interior:10.1186/s12986-016-0101-2. PMC4915038. PMID 27330539.
- Vickers MH (June 2014). "Primaeval life victual, epigenetics and programming of later life disease". Nutrients. 6 (6): 2165–2178. Interior Department:10.3390/nu6062165. PMC4073141. PMID 24892374.
- Chow Q, Li M, Wang X, Li Q, Wang T, Zhu Q, Zhou X, Wang X, Gao X, Li X (2012). "Immune-connected microRNAs are abundant in breast milk exosomes". International Journal of Biological Sciences. 8 (1): 118–123. doi:10.7150/ijbs.8.118. PMC3248653. PMID 22211110.
Extrinsic golf links
| | Wikimedia Commons has media related to messenger RNA. |
- RNAi Atlas: a database of RNAi libraries and their target analytic thinking results
- miRSearch Archived 2012-12-04 at the Wayback Political machine: Puppet for finding microRNAs that target informational RNA
- How mRNA is coded?: YouTube video
- What is mRNA?: theconversation.com
why is an mrna copy made of the dna
Source: https://en.wikipedia.org/wiki/Messenger_RNA
Posting Komentar untuk "why is an mrna copy made of the dna"